Which Of The Following Statements Is True For A System At Chemical Equilibrium?
Which of the following statements is true for a system at chemical equilibrium?. The phosphate ions in calcium phosphate likely react with hydrochloric acid in the stomach to neutralize the pH. When a system that is in equilibrium is disturbed the system adjusts itself to reduce the change. 3 Mg 2 2Al 3Mg 2Al 3 a 10 69 b 10 23.
8 Full PDFs related to this paper. The free energy of formation of nitric oxide NO at 1000 K roughly the temperature in an automobile engine during ignition is about 78 kJmol. Estimate the equilibrium constant for the system indicated at 25 o C.
The concentrations of reactant and products are equal b. Let this be our species vector. When you are done the system will automatically calculate for you the amount you are expected to pay for your order depending on the details you give such as subject area number of pages urgency and academic level.
C The equilibrium partial pressure of Br2 will be greater than 100 atm. After filling out the order form you fill in the sign up details. No player has an incentive to deviate from a Nash equilibrium.
Chair-Elect 2007 Chair 2008 Immediate Past Chair 2009 American Chemical Society Division of Chemical Education. K p 71 10 9. A The equilibrium partial pressures of Br2 Cl2 and BrCl will be the same as the initial values.
V H2 H Br2 Br HBrT the reactions are then defined by Mv where M is a stoichometric matrix in which each row represents a reaction with negative stoichiometric coefficients for reactants and positive stoichiometric coefficients for products. Vectors A B and C are added together as A B C. Ensure you request for assistant if you cant find the section.
A receptor potential is often produced by sensory transduction. Quantitative Chemical Analysis 7E Daniel C.
A Nash equilibrium is a dominant strategy equilibrium.
Which of the following statements regarding Nash equilibrium are correct. Exergy is the energy that is available to be used. Use Q to determine which of the statements below is true. Vice-Chair 2007 and Chair 2009 Gordon. Which Of The Following Statements About Receptor Potentials Is False. Let this be our species vector. It is generally a depolarizing event resulting from inward current flow. Which of the following statements is FALSE. Co-Chair ACS Examinations Institute 2015 General Chemistry Conceptual Exam Committee.
Which of the following are equal for a chemical system at equilibrium. HI 20 M H 2 050 M and I 2 010 M. Chair Biennial Conference Committee Division of Chemical Education American Chemical Society 2014current. As a reaction is moving from reactants to products ie from left to right as written toward equilibrium the value of Q is. After filling out the order form you fill in the sign up details. A The equilibrium partial pressures of Br2 Cl2 and BrCl will be the same as the initial values. Which of the following statements regarding Nash equilibrium are correct.



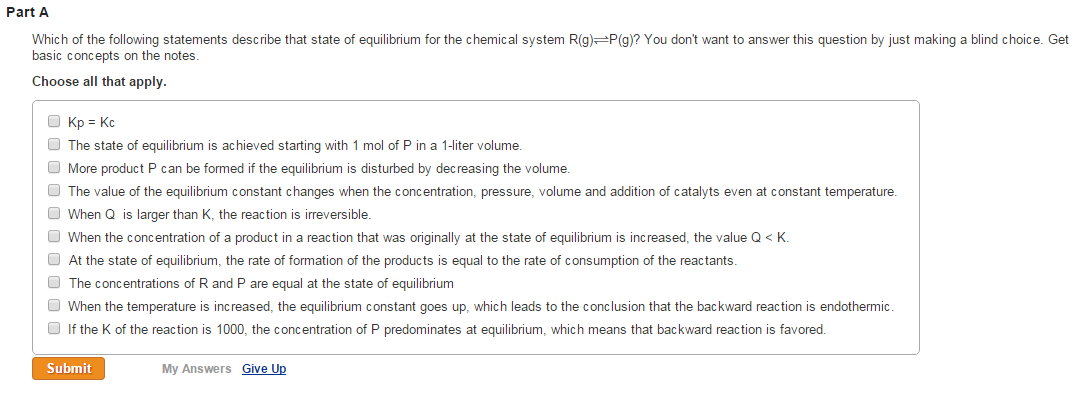
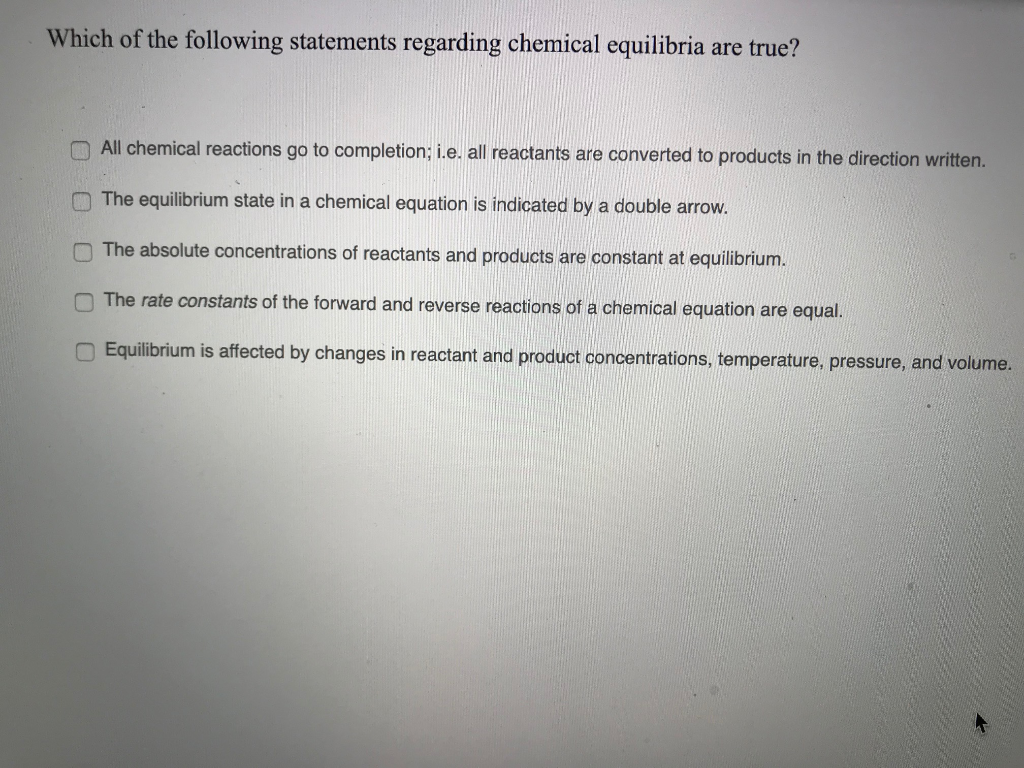




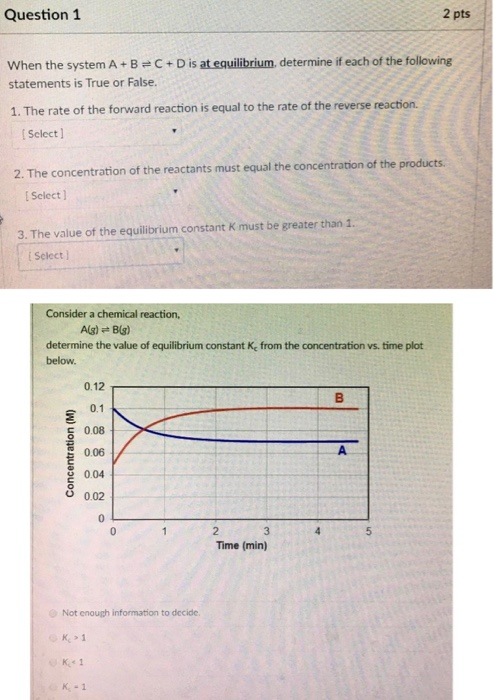







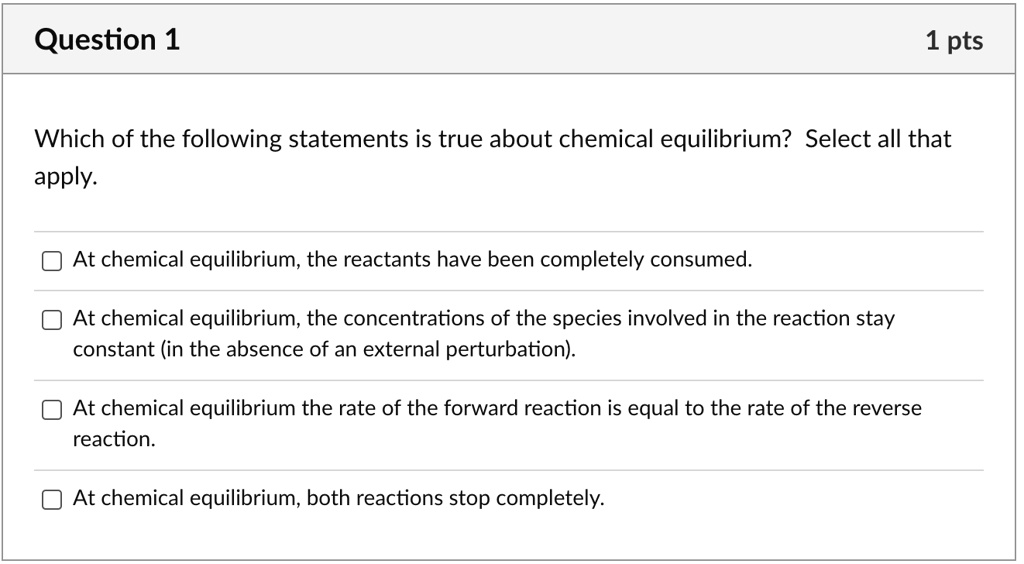





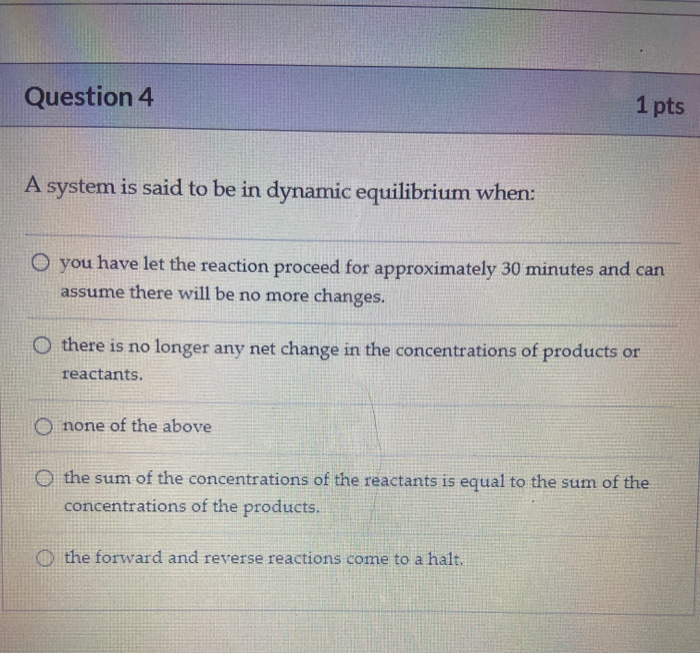






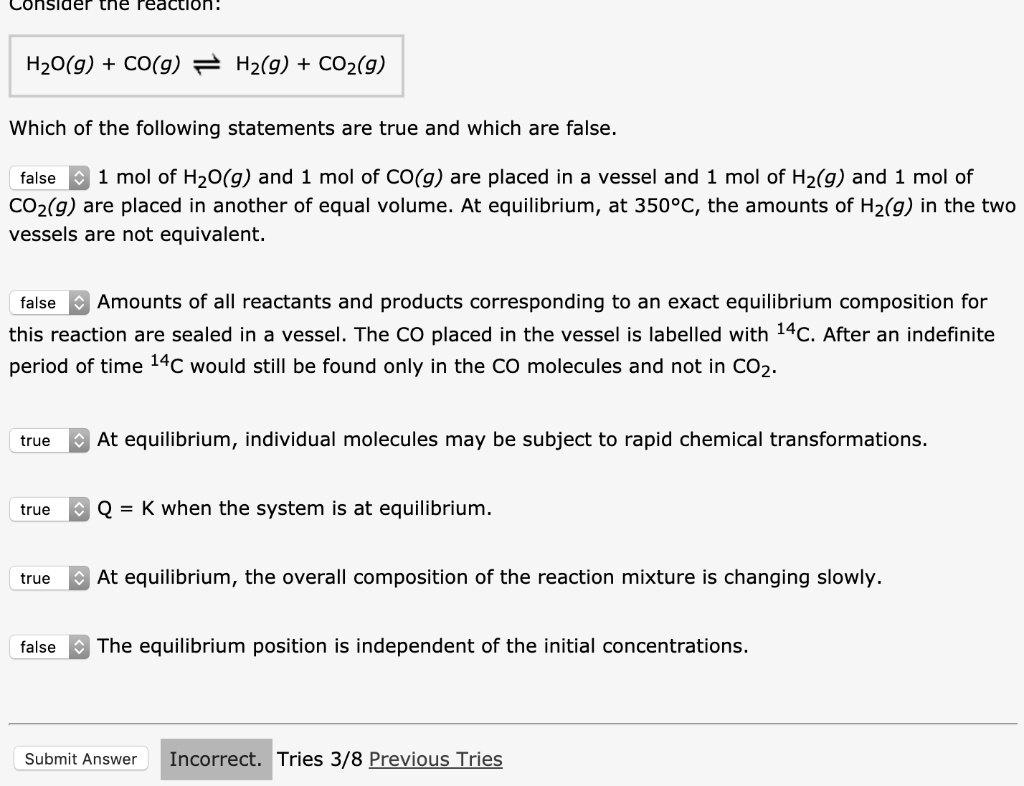


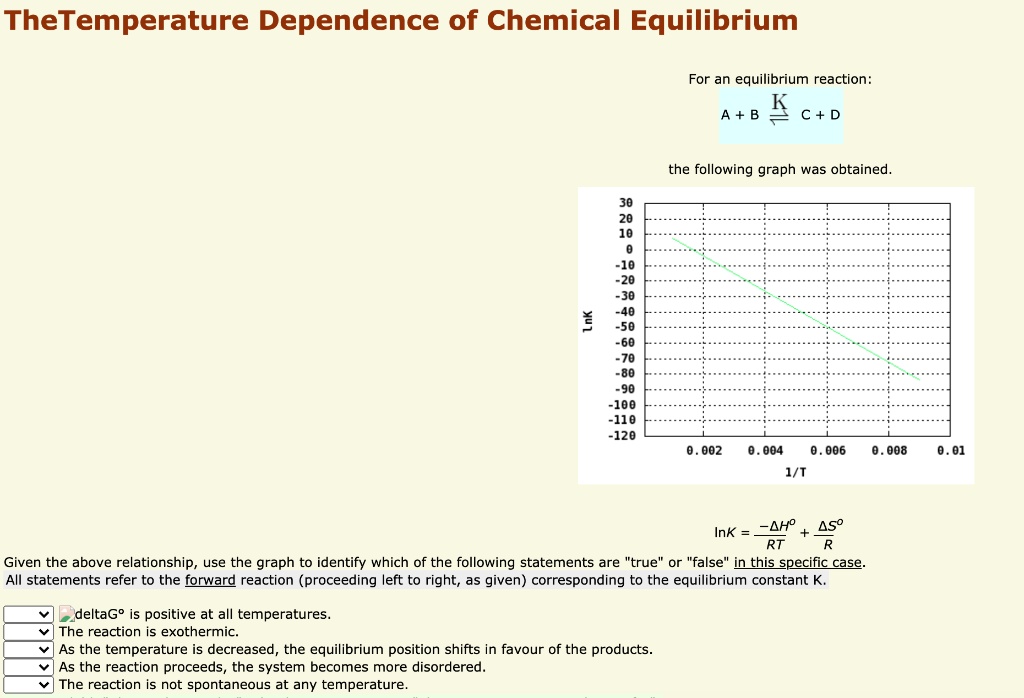




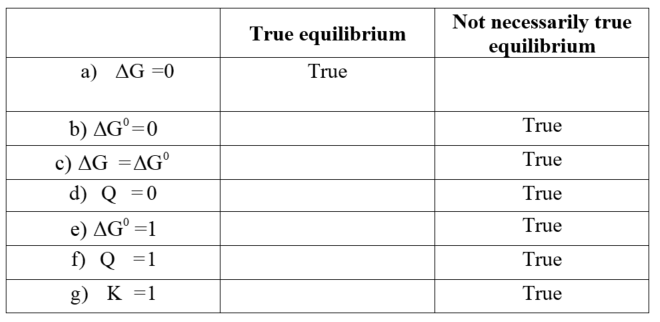









Post a Comment for "Which Of The Following Statements Is True For A System At Chemical Equilibrium?"